Medical device regulation is complex, because of the wide variety of items that are categorized under medical devices. There may be simple tools used during medical examinations, such as tongue depressors and thermometers, or high-tech life-saving devices that are implanted in the patient, like pacemakers and coronary stents. The medical device market has been described as consisting of eight industry sectors: surgical and medical instrument manufacturing, surgical appliance and supplies, electromedical and electrotherapeutic apparatus, irradiation apparatus, ophthalmic goods, dental equipment, and supplies. The federal agency responsible for regulating medical devices is the Food and Drug Administration (FDA)—an agency inside the Department of Health and Human Services. A manufacturer must gain FDA’s prior approval or clearance before realizing any medical instrument.
Food and Drug Administration has standards to be followed for integrated quality systems and risk management approaches into its existing programs with the specific goal of encouraging industry to adopt modern and innovative manufacturing technologies.
- FDA Devices Classification:
FDA (U.S. Food and Drug Administration) has mentioned on his web all the rules which are needed to be followed. They classify devices on the basis of risk. Lower risk devices are exempted from the procedure. Moderate risk devices are cleared for marketing if they are substantially equivalent to the already marketed devices. However, highest-risk devices such as infusion pump require approval before marketing, and FDA expects a reasonable assurance of safety and effectiveness for them. That’s where the QA comes in the picture – after all, it’s all about assurance!!!
A very critical yet responsible job for QA!! While thinking about FDA approvals, bulletproof QA execution comes first in the as the products are going to be used on human-beings like us. Indeed, very crucial, and that’s why it’s essential to have product approvals.
- Quality Assurance Execution Cycle for FDA Approval of Medical Devices:
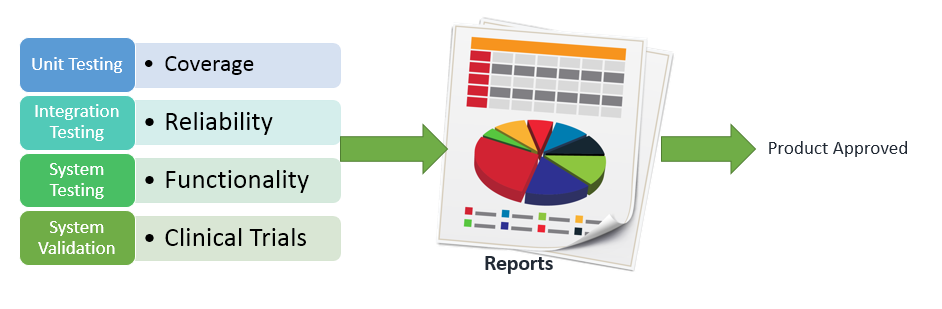
Well, the industries have grown very far in QA, and with years, experts have come up with the automation test frameworks such as VectorCast to ease the validations. Software like that facilitates to perform efficient execution of unit, integration and system testing. Wherein the purpose of unit testing is to validate code in small segments and cover maximum possible lines; integration testing or compound testing is to verify reliability at internal and external interfaces and check feasibility by grouping multiple modules; and system testing is to validate the functionality level scenarios including load testing, stress testing, performance testing, security testing and whatever needed for the respective product.
- VectorCast: Recommended Tool for The Best Practices of Quality Assurance
VectorCast comes in the multiple variants, and each of these has its own significance.
- VectorCast/C++: It is helpful in unit testing, and it offers maturity to scan the software and generate test-cases by its own, which is very helpful to perform any QA task.
- VectorCast/Cover: It helps in identifying the code that is covered by the test-cases defined with VectorCast/C++, and most importantly it also marks the region which isn’t covered which helps a QA person in work-out for having 100% coverage of the code.
- VectorCast/Manage: It comes with the huge support of regression, which facilitates QA person to queue the test-cases and define a frequency for single or multiple executions.
After completing all the technical testing, the product has to undergo clinical trials for the real time assurance. And once all the procedures are completed, the reports will be submitted to FDA for the approval. Yeah, the process of approval can be different as per authority but, for QA, the battle remains the same!!
VOLANSYS have the backbone of expertise in that direction. In one of the decent case studies, we have done Quality Engineering for infusion pump by ensuring it meets all the relevant medical standards. Our experts delivered Unit testing, Functional testing, Integration testing System testing complying FDA and HIPPA protocols.
If you have medical devices and looking for an assistance to get it approved by FDA, then a solid QA execution is only a click away. Click here.

About the Author: Aalok Shah
Aalok Shah is working in VOLANSYS Technologies as a Sr. Engineer. He has served in multiple industry verticals and worked upon many tools and technologies till now in his journey. Being passionate, he always looks forward to the opportunities to bring better solutions on the table.